Abstract
Background: The prognosis of refractory/relapsed aggressive B-cell non-Hodgkin lymphoma (r/r B-NHL) is extremely poor especially for the patients who failed to CD19-Specific chimeric antigen receptor-T (CAR-T) cells therapy.Data guiding management of this challenging population are lacking.
Aims:We performed a retrospective analysis with a primary objective to assess salvage strategies and outcomes following CD19 CAR-T failure.
Methods:From December 2019 to May 2022, 96 patients who had failed therapy with anti-CD19 CAR -T cells were included.Median age was 49 (13-74) years. Diagnoses included DLBCL NOS (n=65) ,PMBCL(n=8) ,TFL(n=7), BL (n=9),and Other(n=7). In the primary cohort, 84/96 (87.5%) patients were at stage III-IV at the time of salvage therapy. The median IPI score was 3 (range 1-5). 75/96 (78.1%) patients had extranodal lesions. 43/96(44.8%)patients had>7cm bulky disease and 18/96(18.8%)patients failure of prior autologous hematopoietic stem cell transplantation (HSCT).34/96(35.4%)cases were with TP53 mutations.Prior to the study, CD20/CD22/CD79b antigen expression in tumour tissue was confirmed by pathology, and the target was selected according to antigen expression.
Salvage strategies after CD19 CAR-T failure include sequential CAR-T cell therapy with a different target and combined chemotherapy. To further reduce tumour burden, bridging therapy (BT) has been approved prior to sequential CAR-T cell therapy with another target.
The kinetics and function of CAR-T cells were monitored by quantitative PCR and flow cytometry. Efficacy was assessed by PET-CT every 3 months after salvage therapy. All p-values were two-sided values. Survival curves were calculated by the Kaplan-Meier method.
Results: CD19 antigen status was determined in 38/96 patients (39.6%), with 11/38 (28.9%) having CD19-negative relapse. Data from 78/96 patients could be included in the analysis. 48/78 (61.5%) of patients were selected for subsequent secondary CAR-T cell therapy and 30/78 (38.5%) for combined chemotherapy at a median of 102 days (26-323) after CD19 CAR-T infusion.The number of patients selecting CD20 target and CD22 target for secondary CAR-T treatment was 34/48 (70.8%) and 14/48 (29.2%), respectively. The median secondary CAR-T cells subsequently infused were 1.57 (range, 0.56-5.66)×106/kg. A grade 3 or higher cytokine release syndrome occurred in 7/48 (14.6%), and grade 3 or higher neurologic events occurred in 4/48 (8.3%).
A response occurred in 10/48 (20.8%) of patients in the subsequent secondary CAR-T cell therapy and in 3/30(10%)of patients in the combined chemotherapy group (with a complete response in 7/10 and 2/3,respectively).By Fisher's precision probability test, patients with>7cm bulky disease (P=0.0125) and those with TP53 gene mutations (P=0.0115) had worse outcomes with salvage therapy. A significantly lower peak level of secondary CAR-T cells was observed in patients with no effect compared with patients who had a durable response (P=0.0403).
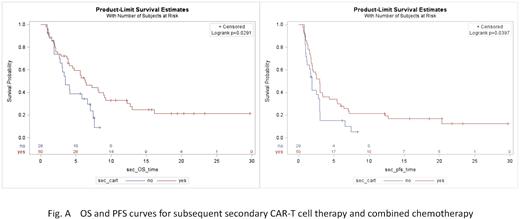
With a median follow-up of 18.51 months (95% CI: 11.80-21.44), the 3-month progression-free survival (PFS) (48.0% vs. 34.4%, P=0.0397) and 6-month overall survival (OS) (53.1% vs. 34.52%, P=0.0291) were even longer in the group followed by secondary CAR-T cell therapy than in the group with combination chemotherapy. Fig. A shows the survival probability after treatment.
The three prognostic factors comprising the OS prognostic tool: time interval between two treatments with CAR-T cell therapy>100 days apart (P=0.0392), >7cm bulky disease before salvage therapy (P=0.0493), and having TP53 gene mutations (P=0.0256). The addition of polatuzumab-based therapies did not improve outcomes or survival in the BT before subsequent secondary CAR-T or in chemotherapy groups.Up to the date of follow-up,36/78(46.2%) patients died from disease progression.No deaths related to cytokine release syndrome or neurologic events occurred.
Conclusions: Our data suggest that subsequent secondary CAR-T cell therapy was superior to chemotherapies as a safe and effective salvage strategy for CAR19-refractory B-cell lymphomas. However, studies on curative approaches and long-term follow-up are needed.
[Key words] refractory/relapsed B-cell lymphoma;Failure of anti-CD19 CAR-T Cell therapy ;Subsequent secondary CAR-T cell threapy;Polatuzumab-based therapies
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal